First brain images presented at ECCN
NeuroLF brain images presented first time
April 4, 2024
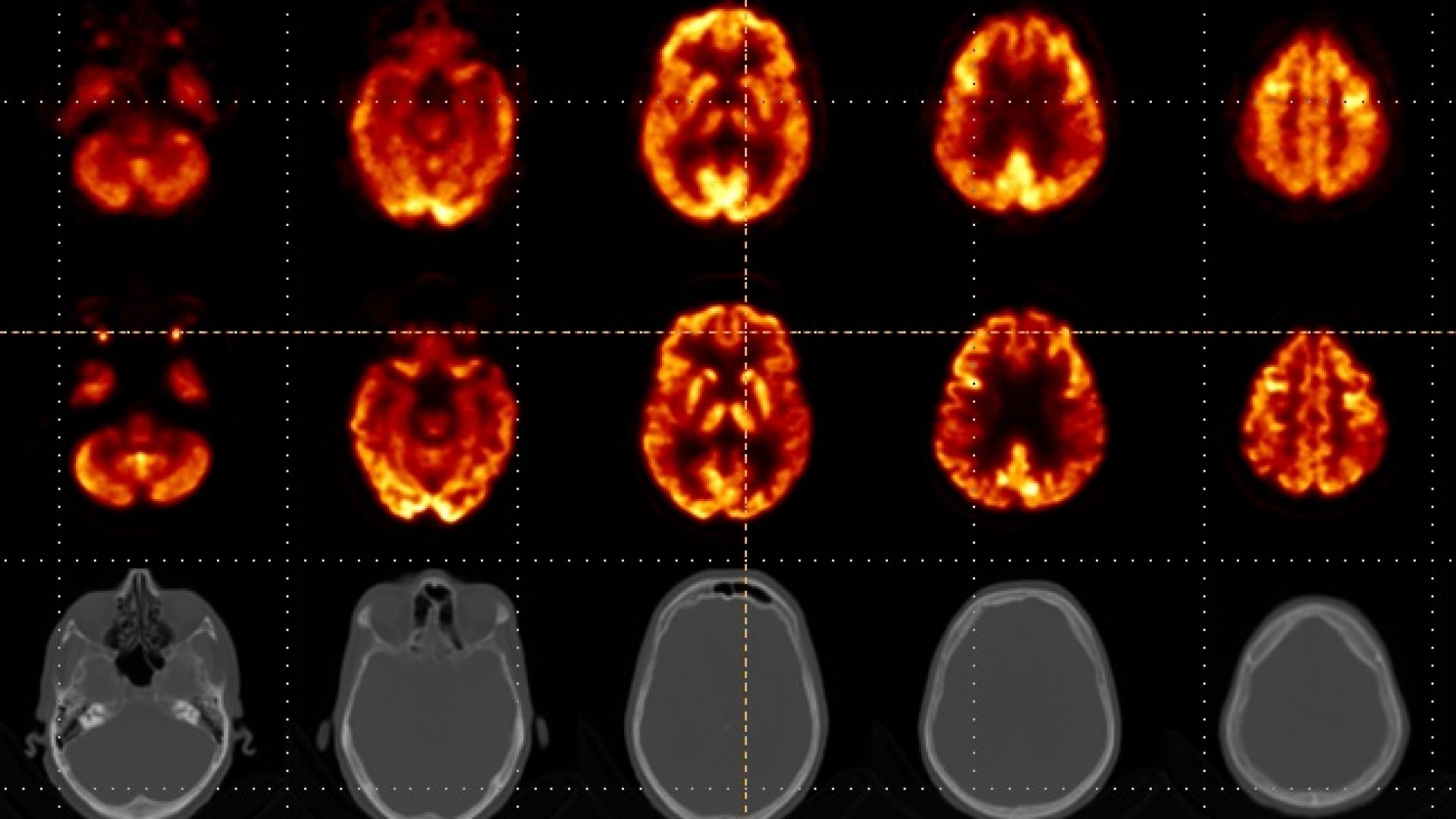
First brain images of the NeuroLF system were presented at the European Conference on Clinical Neuroimaging (ECCN) in Leipzig, Germany last week.
Positrigo, a Swiss based company developing nuclear medical imaging devices to advance functional brain imaging, reports the start of a clinical trial and presents the first brain images of the NeuroLF system. NeuroLF is an ultra-compact and patient-centric designed brain PET system which assists in diagnosing and monitoring of brain related disorders like Alzheimer’s disease, Brain Tumors, Epilepsy, Parkinson’s disease and others. The system is not yet commercially available.
Since March, the dedicated brain PET system NeuroLF has been successfully used in a study at the University of Leipzig Medical Center. The goal of the study, which will eventually expand to include also the University Hospital Zürich, is to compare the device with conventional PET/CT and PET/MR devices. Just last week, the first official brain images were presented at the European Conference of Clinical Neuroimaging (ECCN). The analysis of these preliminary images shows clinical equivalency compared to the quality of images from a full body PET/CT device. The principal investigator of this study, nuclear medicine expert Univ.-Prof. Dr. med. Henryk Barthel assessed the first preliminary results and concluded: “After analyzing the first NeuroLF brain images, I am impressed by their quality. The images show non-inferiority compared to conventional PET/CT devices and even in comparison with the Biograph Vision Quadra from Siemens – considered a best-in-class device – they look good”. Positrigo’s Chief Technology Officer, Dr. Ilaria Sacco, is also satisfied with these first images: “The entire team worked very hard and it is therefore extremely rewarding to see how NeuroLF is performing and what clinical impact the device can have in the future”.
“After analyzing the first NeuroLF brain images, I am impressed by their quality. The images show non-inferiority compared to conventional PET/CT devices and even in comparison with the Biograph Vision Quadra from Siemens they look good.”
Univ.-Prof. Dr. med Henryk Barthel
University of Leipzig Medical Center, Department of Nuclear Medicine (Director and Chairman Univ.-Prof. Dr. Osama Sabri)

Increased Demand for Brain PET
The development of a dedicated brain PET device is very timely as just last year the first disease-modifying therapies for Alzheimer’s Disease (AD) have been approved in the US1. AD is the most common form of dementia, accounting for ~70% of all dementias in those over 60. In 2020 there were over 55 million people worldwide living with dementia and it is predicted that individuals affected by AD will grow to 139 million in 20502. The early diagnosis is of outmost importance and PET imaging of the brain is essential for the accurate diagnosis of AD patients. However, the currently available PET scanners are large and always combined with MRI or CT. The purchase and maintenance costs are significant and in addition a lot of space is required which limits the availability of these devices. “Especially in the US we experience a lot of interest in our technology and can’t wait to receive market clearance from the FDA which we expect in the coming months”, states Dr. Jannis Fischer, co-founder and CEO of Positrigo.
About Positrigo:
Positrigo is a pioneer in nuclear medical imaging technologies. Headquartered in Zurich, Switzerland, the medical device company was founded in 2018 as a spin-off of ETH Zurich. Positrigo’s technology, development, clinical testing and commercialization has been supported by various private investors, the Swiss government and the European Innovation Council. NeuroLF – the company’s first device – is an ultra-compact brain Positron Emission Tomography (PET) scanner which has applications in assisting the diagnosis of causes of dementias, such as Alzheimer’s disease and other brain related disorders.
References: